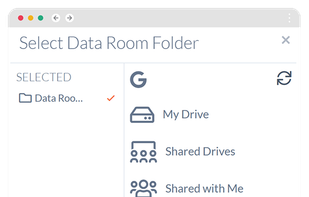
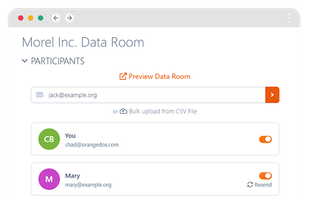
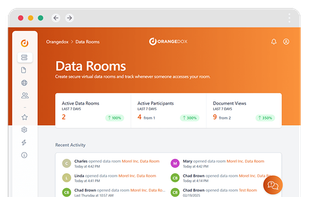
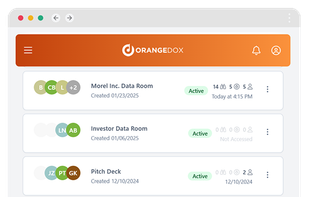
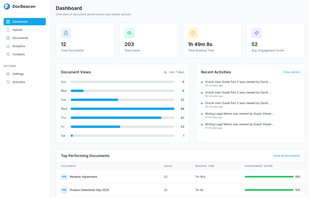
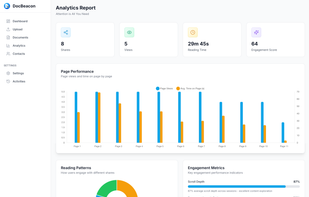
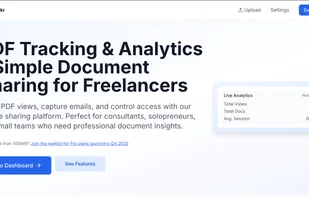
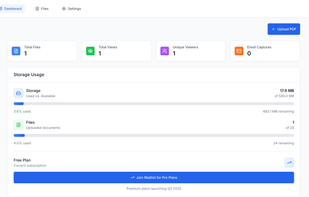
Orangedox provides one-click virtual data rooms that are directly synced with your Google Drive folders. Prevent your documents from being shared or forwarded and audit every time they've been opened.
Cost / License
- Paid
- Proprietary
Application types
Platforms
- Online
- Software as a Service (SaaS)
- Google Workspace