Cruxi
Cruxi is a web-based AI platform that helps medical device teams and regulatory consultants plan, draft, validate, and package FDA 510(k) submissions faster.
Cost / License
- Pay once or Subscription
- Proprietary
Platforms
- Online
Features
- Workflow Management
Tags
- Compliance
- regulatory-affairs
- medical-devices
- consultants
Cruxi News & Activities
Recent activities
- Cruxi added Cruxi
 Cruxi added Cruxi as alternative to Drugs.com, WebMD, Medicamentum and Offline Drug Dictionary : Free - Medical
Cruxi added Cruxi as alternative to Drugs.com, WebMD, Medicamentum and Offline Drug Dictionary : Free - Medical
Cruxi information
What is Cruxi?
Cruxi is a web-based AI platform that helps medical device teams and regulatory consultants plan, draft, validate, and package FDA 510(k) submissions faster.
Key capabilities include:
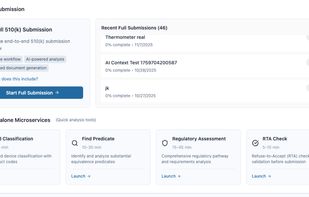
510(k) drafting support aligned to common submission sections and best practices
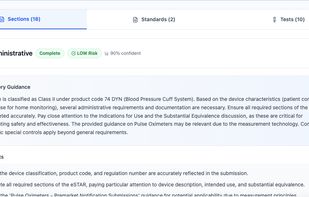
Device pathway and requirements guidance (with structured knowledge from FDA sources)
Predicate discovery and side-by-side comparison tools
Change impact and modification analysis for existing devices
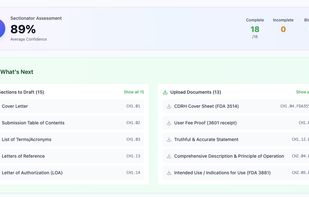
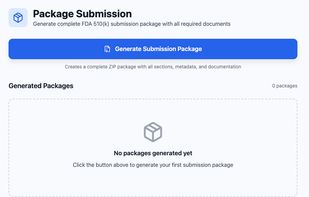
eSTAR package checks and submission validation workflows
Consultant directory to match startups with experienced 510(k) reviewers
Cruxi is built for regulatory professionals who want to reduce manual research and formatting work while keeping experts in control of the final submission
Cruxi also maintains the biggest directory of FDA 510K Consultants https://cruxi.ai/pages/regulatory/fda-510k-consultant.html